United States In Vitro Diagnostics Market Overview
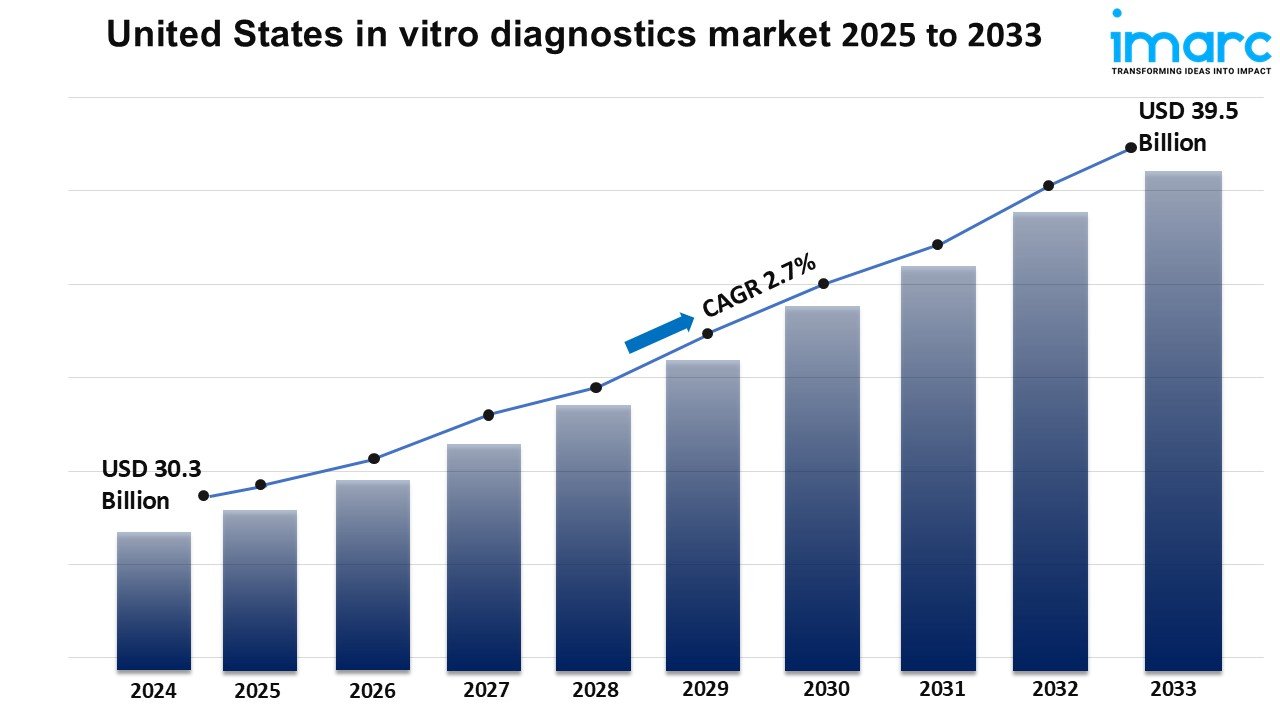
Market Size in 2024: USD 30.3 Billion
Market Forecast in 2033: USD 39.5 Billion
Market Growth Rate (2025-2033): 2.7%
The United States in vitro diagnostics market size was valued at USD 30.3 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 39.5 Billion by 2033, exhibiting a CAGR of 2.7% from 2025-2033.
United States In Vitro Diagnostics Market Trends and Drivers:
The United States in vitro diagnostics market is increasing steadily, propelled through the growing burden of continual ailments along with diabetes, cardiovascular disorders, and cancer. Healthcare carriers are constantly counting on IVD technology to permit early detection, particular sickness management, and tailor-made remedy regimens, thereby improving affected person outcomes. This growing call for for well timed and dependable diagnostic insights is aligning with the wider shift towards customized medicine, that’s reshaping scientific practices nationwide. Simultaneously, ongoing improvements in molecular diagnostics, immunoassays, and point-of-care trying out are optimizing diagnostic efficiency, accuracy, and accessibility. Laboratories and hospitals are integrating those superior gear into habitual scientific workflows, responding to a developing populace of sufferers requiring common and speedy diagnostic evaluation. As a result, the IVD area is positioning itself as an essential pillar of cutting-edge U.S. healthcare infrastructure.
Technological improvements continue to be a center catalyst for marketplace growth, as corporations are constantly growing automatic structures and AI-incorporated diagnostic structures that streamline laboratory operations and limit human error. In addition, the developing adoption of at-domestic trying out kits is reworking affected person engagement through making diagnostics greater client-centric. These improvements aren’t best enhancing trying out accessibility throughout city and rural areas however additionally fostering a tradition of preventive healthcare. Within the United States, favorable authorities rules assisting diagnostic research, mixed with a robust healthcare compensation framework, are strengthening the industry`s foundation. Furthermore, the increasing aged populace greater liable to continual and infectious diseases is maintaining regular call for for diagnostic services. Industry leaders are capitalizing on those possibilities thru strategic partnerships, product innovation, and growth of distribution channels, making sure long-time period marketplace sustainability and competitiveness.
In this dynamic environment, the U.S. in vitro diagnostics marketplace is likewise profiting from growing scientific trial pastime and growing healthcare expenditure, each of which might be encouraging the non-stop evolution of diagnostic standards. Regulatory government are expediting approvals for novel diagnostic assays, similarly accelerating marketplace access and using innovation. Additionally, the mixing of virtual fitness technology, which includes cloud-primarily based totally records sharing and telemedicine, is raising the price proposition of diagnostic solutions. As healthcare carriers prioritize early prognosis and customized care, the IVD marketplace is rising as a crucial enabler of scientific decision-making. With a strong pipeline of diagnostic improvements and robust call for from each scientific and client segments, the marketplace is retaining a trajectory of sustained increase and strategic growth.
For an in-depth analysis, you can refer sample copy of the report:
https://www.imarcgroup.com/united-states-in-vitro-diagnostics-market/requestsample
United States In Vitro Diagnostics Market Industry Segmentation:
Analysis by Test Type:
- Clinical Chemistry
- Molecular Diagnostics
- Immunodiagnostics
- Hematology
- Others
Analysis by Product:
- Reagents and Kits
- Instruments
Analysis by Usability:
- Disposable IVD Devices
- Reusable IVD Devices
Analysis by Application:
- Infectious Disease
- Diabetes
- Cancer/Oncology
- Cardiology
- Autoimmune Disease
- Nephrology
- Others
Analysis by End User:
- Hospitals Laboratories
- Clinical Laboratories
- Point-of-care Testing Centers
- Academic Institutes
- Patients
- Others
Regional Analysis:
- Northeast
- Midwest
- South
- West
Competitive Landscape:
The competitive landscape of the industry has also been examined along with the profiles of the key players.
Ask Our Expert & Browse Full Report with TOC & List of Figure:
https://www.imarcgroup.com/request?type=report&id=11158&flag=C
Key highlights of the Report:
- Market Performance (2019-2024)
- Market Outlook (2025-2033)
- COVID-19 Impact on the Market
- Porter’s Five Forces Analysis
- Strategic Recommendations
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Structure of the Market
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
Note: If you need specific information that is not currently within the scope of the report, we can provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145