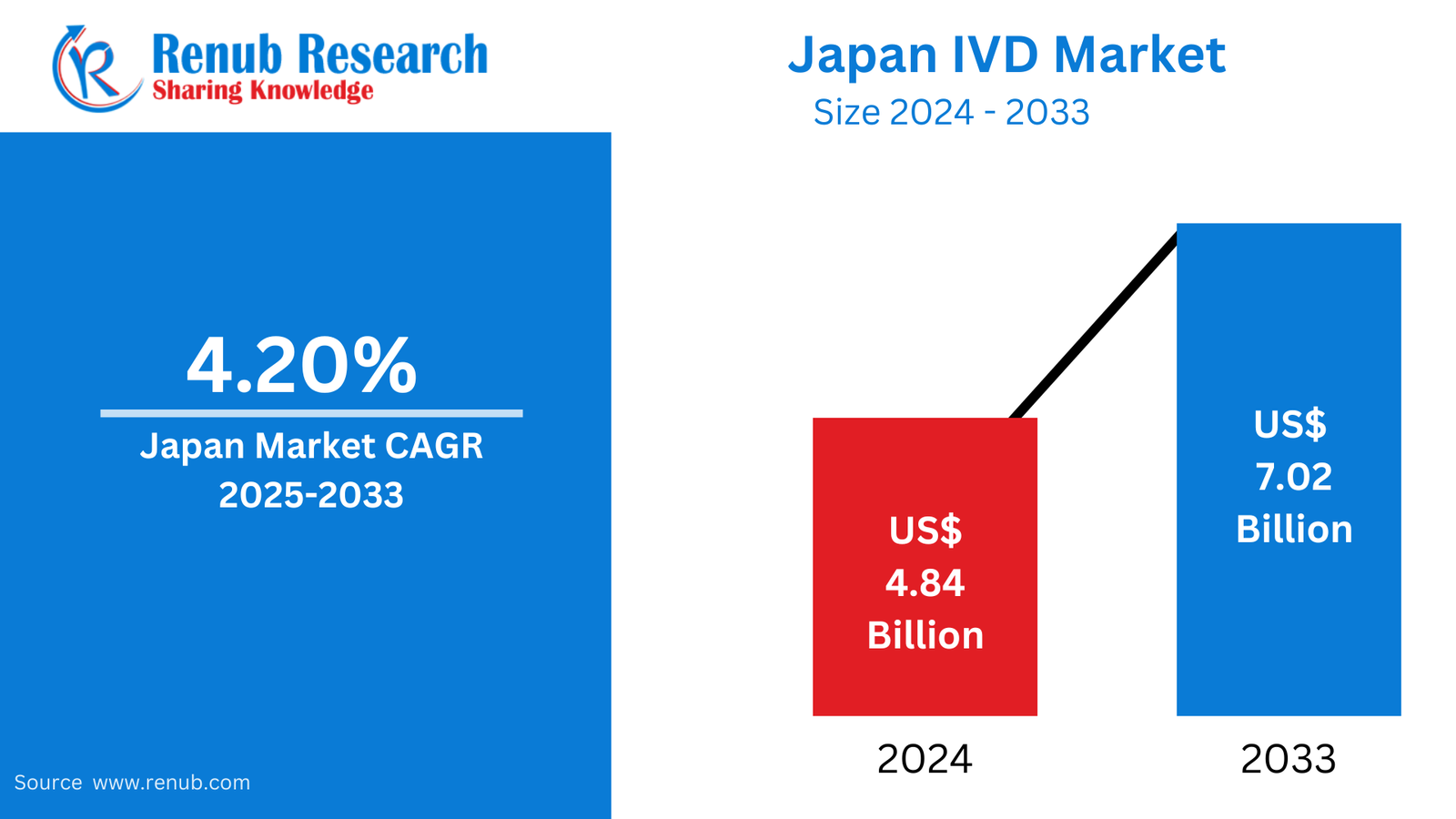
According to the latest report by Renub Research, the Japan In-Vitro Diagnostics (IVD) Market is projected to experience substantial growth during the forecast period 2024–2032, driven by increasing demand for early and accurate disease detection, a rapidly aging population, and advancements in molecular diagnostics and personalized medicine. Japan, recognized for its high healthcare standards and cutting-edge technology, presents a fertile ground for IVD companies seeking growth opportunities in Asia.
📊 Explore the Full Report Here: Japan In-Vitro Diagnostics Market Forecast 2024–2032
Demographic Drivers: Japan’s Aging Society Creates Sustained Demand
Japan has the oldest population globally, with over 28% of its citizens aged 65 and older. This demographic trend leads to a higher prevalence of age-related conditions, such as cancer, cardiovascular diseases, diabetes, and infectious diseases. The increasing disease burden necessitates routine and advanced diagnostics, positioning in-vitro diagnostics as a cornerstone of Japan’s healthcare system.
IVD tests allow physicians to detect diseases at an early stage and monitor ongoing conditions with precision and speed. This is especially critical in a super-aged society where proactive health management is not only essential for improving quality of life but also for reducing the overall cost burden on the national healthcare system.
Key Market Drivers Accelerating IVD Growth in Japan
1. Technological Advancements and Automation
The Japanese IVD market is benefitting from innovations in diagnostic technologies such as point-of-care testing (POCT), liquid biopsy, next-generation sequencing (NGS), digital pathology, and high-throughput molecular testing. Automated diagnostic platforms are gaining traction in hospital and laboratory settings, enhancing efficiency and reducing human error.
2. Expanding Role of Molecular Diagnostics
Molecular diagnostics is one of the fastest-growing segments in the IVD industry, driven by its high specificity and sensitivity. These diagnostics are widely used for detecting infectious diseases like COVID-19, influenza, and hepatitis, as well as in cancer screening and pharmacogenomics. Japan’s highly developed pharmaceutical sector is fostering companion diagnostics to enable personalized therapies.
3. COVID-19 Impact and Ongoing Infectious Disease Surveillance
The COVID-19 pandemic significantly expanded testing infrastructure in Japan. While the pandemic accelerated the adoption of PCR and antigen testing, it also increased public and institutional awareness of infectious disease surveillance, likely leading to permanent structural demand in the IVD sector even in the post-pandemic world.
4. Government Initiatives and Healthcare Reforms
Japan’s Ministry of Health, Labour and Welfare (MHLW) continues to support medical innovation and is actively promoting preventive healthcare. Policies aimed at integrating AI and digital health solutions into diagnostics, as well as reimbursement support for advanced testing methods, are boosting the IVD market.
5. Rising Demand for Home and Point-of-Care Testing
With an increase in remote patient monitoring and home-based healthcare services, point-of-care tests (POCT) and self-administered diagnostics are gaining popularity in Japan. These tests enhance accessibility and reduce diagnostic delays, especially in rural and aging communities.
Market Segmentation: Diverse Applications of IVD
The Japan In-Vitro Diagnostics Market is segmented by technology, application, end-user, and product type. Each segment plays a crucial role in the healthcare continuum.
By Technology:
- Clinical Chemistry
- Immunoassay
- Hematology
- Molecular Diagnostics
- Coagulation
- Microbiology
- Point-of-Care Testing
Molecular diagnostics and immunoassay technologies are forecasted to witness the most significant growth due to their applications in chronic and infectious disease detection.
By Application:
- Infectious Diseases
- Oncology
- Cardiology
- Nephrology
- HIV
- Diabetes
- Autoimmune Diseases
The oncology and infectious disease segments are expected to dominate due to a growing focus on early cancer detection and epidemic preparedness.
By End-User:
- Hospitals
- Laboratories
- Homecare Settings
- Academic and Medical Institutions
Hospitals and diagnostic laboratories remain the largest consumers of IVD services, though homecare settings are growing rapidly due to Japan’s home-based elder care model.
Competitive Landscape: Innovation and Localization are Key
Japan’s IVD market is characterized by a mix of domestic giants and international players. Leading companies are focusing on innovation, strategic partnerships, and localization to gain market share.
Key Players Include:
- Sysmex Corporation
- Roche Diagnostics
- Abbott Laboratories
- Siemens Healthineers
- Thermo Fisher Scientific
- Fujirebio (H.U. Group Holdings)
- Becton Dickinson and Company (BD)
- Bio-Rad Laboratories
- Beckman Coulter
Domestic firms like Sysmex are leading in hematology and laboratory automation, while international companies dominate molecular diagnostics and immunoassay segments.
Market Challenges: Navigating Regulation and Cost Pressures
Despite a positive growth outlook, the Japan IVD market faces certain barriers:
- Regulatory Hurdles: Japan’s medical device regulatory framework requires meticulous approvals from PMDA (Pharmaceuticals and Medical Devices Agency), often leading to longer time-to-market.
- Reimbursement Constraints: Cost-containment pressures from the government occasionally limit reimbursement for newer tests, impacting adoption rates.
- High Market Competition: Established players dominate major IVD segments, creating entry challenges for new and smaller firms.
However, these challenges are being gradually addressed through regulatory harmonization and increased collaboration between government bodies and industry stakeholders.
Future Outlook: Toward Personalized and Predictive Healthcare
Japan’s IVD landscape is moving toward precision healthcare, where diagnostics play a central role in determining the right treatment for the right patient at the right time. Artificial intelligence, real-time health monitoring, wearable diagnostic sensors, and multi-omics data integration will redefine the next generation of diagnostic services.
Conclusion: Japan a Key Growth Frontier for IVD
The Japan In-Vitro Diagnostics Market offers robust growth potential, backed by favorable demographics, a strong healthcare infrastructure, and continuous innovation in diagnostics. Companies investing in research, localization, and digital integration stand to benefit the most.
To know more about this growing market, access the full research insights here: Japan In-Vitro Diagnostics Market Report
New Publish Report:
- United States Automotive Air Suspension Market – Vehicle Trends & Forecast 2025–2033
- United States Orthodontics Market – Treatment Trends & Forecast 2025–2033
- United States Smart Pills Market – Drug Delivery Innovation & Forecast 2025–2033
About the Company
Renub Research is a Market Research and Consulting Company with more than 15 years of experience, especially in international Business-to-Business Research, Surveys, and Consulting. We provide a wide range of business research solutions that help companies make better business decisions. We partner with clients across all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
Our wide clientele includes key players in Healthcare, Travel & Tourism, Food & Beverages, Power & Energy, Information Technology, Telecom & Internet, Chemicals, Logistics & Automotive, Consumer Goods & Retail, Building & Construction, and Agriculture. Our core team comprises experienced professionals with graduate, postgraduate, and Ph.D. qualifications in Finance, Marketing, Human Resources, Bio-Technology, Medicine, Information Technology, Environmental Science, and more.
Media Contact
Company Name: Renub Research
Contact Person: Rajat Gupta, Marketing Manager
Phone No: +91-120-421-9822 (IND) | +1-478-202-3244 (USA)
Email: rajat@renub.com